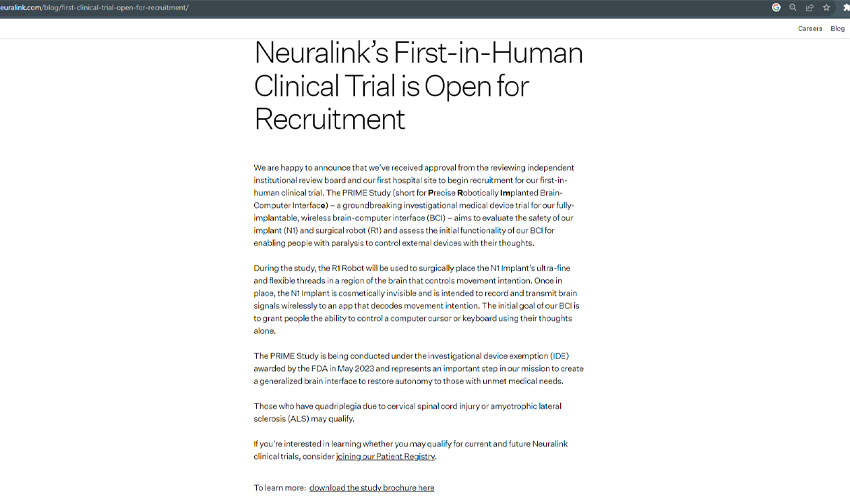
Neuralink Corp., the groundbreaking brain implant startup led by tech mogul Elon Musk, has taken a significant step toward making science fiction a reality by commencing patient recruitment for its first-ever human clinical trial.
In an official blog post, Neuralink announced its intention to recruit patients dealing with quadriplegia resulting from cervical spinal cord injuries or amyotrophic lateral sclerosis (ALS) for this groundbreaking trial.
The primary objective is to assess the safety and functionality of Neuralink's technology, allowing individuals to control external devices solely with their thoughts.
The initial aim of the trial is to empower participants with the ability to manipulate a computer cursor or keyboard through their thoughts alone.
While Musk has often discussed ambitious long-term goals for Neuralink, like enabling language learning through brain implants or mental communication, the company has consistently emphasized its commitment to addressing brain injuries as its first major endeavour.
Neuralink's human trial received early approval from the Food and Drug Administration (FDA) in May, specifically through an investigational device exemption.
This regulatory milestone enables the company to proceed with human trials. Neuralink also confirmed receiving approval from the hospital where the initial surgeries will be performed, although the hospital's name remains undisclosed.
Though the initiation of this human clinical trial is a significant achievement for Neuralink and the field of brain implants, it is important to note that the path to broader adoption and further trials will likely be lengthy, as acknowledged by experts in the field.
Victor Krauthamer, former director of the division of Biomedical Physics at the FDA, emphasized that such developments typically entail years of rigorous testing and evaluation.



























